Home / Knowledge hub /
Optimizing spray formation of low GWP metered dose inhalers – unlocking product performance understanding

by Ben Myatt
November 27, 2023
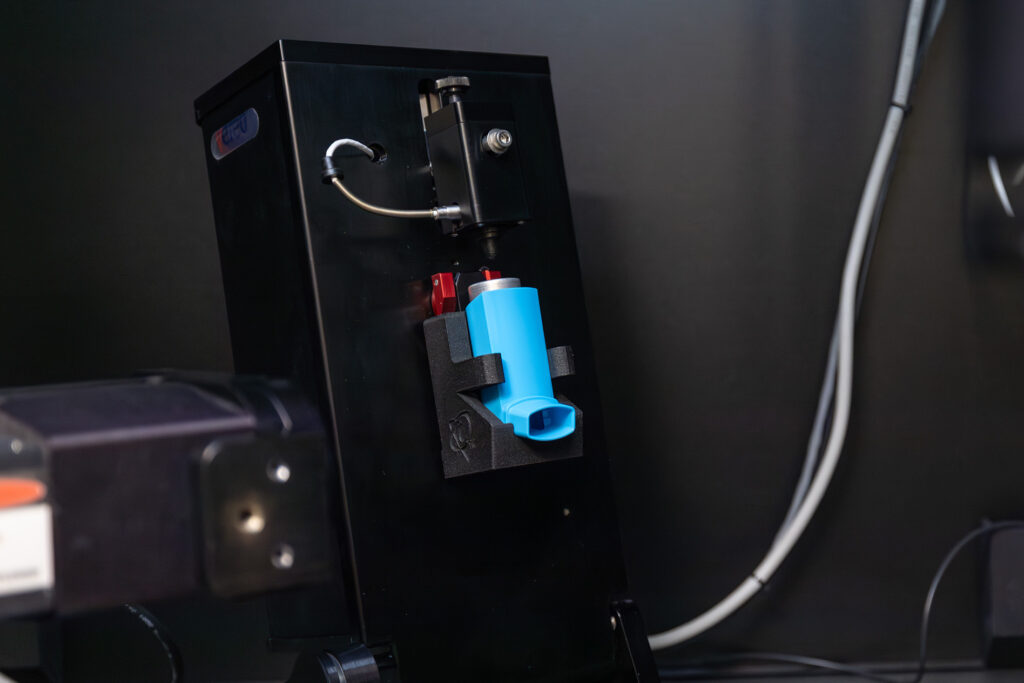
Thank you for attending the webinar for Benjamin Myatt, R&D Staff Scientist at Kindeva, and Daniel Duke, Senior Lecturer in the Department of Mechanical & Aerospace Engineering at Monash University. This session explored key issues related to spray formation and product development for MDI using green propellants.
Key Takeaways:
- The differences in pMDI propellant properties and implications of low-GWP options for droplet production and product performance
- A range of investigations undertaken, including novel high-speed imaging and X-ray diagnostics and pharmacopeial performance experiments, to explore the impact of switching to green propellants in pMDI products
- A summary of key findings and insights to enable tuning of low-GWP pMDI product performance
Download The Presentation
Fields marked with a * are required.
About the author

Related resources
Explore our other resources to discover valuable insights on the latest trends in drug delivery.
HFA152a green propellant capabilities
With evolving regulations and a growing push for sustainability, switching pressurized metered-dose inhalers (pMDIs) to green propellants is becoming increasingly essential. One of the most promising low global warming potential (GWP) options is HFA152a. In this white paper, we share insights on transitioning to HFA152a. Download your copy to discover: An overview of sustainability regulations […]
Learn MoreLeading the way in low-GWP propellants
As regulations surrounding inhalation device sustainability evolve, we help you streamline the process of transitioning to low global warming potential (GWP) propellants. Clinical supply for green propellants HFA152a and HFO1234ze is already available, with commercial scale available this year and plans for further expansion. Download this information sheet to dive deeper into our low-GWP propellant […]
Learn MoreOpportunities and considerations for inhaled biologics
Respiratory biologics is an emerging area in pharmaceutical development, accounting for approximately 10% of biologics currently in the pipeline. These innovative treatments offer significant benefits across various therapeutic areas. Gain deeper insights into the development of inhaled biologics with this comprehensive presentation by experts from Kindeva Drug Delivery, Aerogen Pharma, and Cystetic Medicines. Highlights include: […]
Learn MorePulmonary & nasal delivery capabilities
Get an overview of our pulmonary & nasal delivery capabilities, including: Customized approach to determining the right device for your drug Pulmonary & nasal platform technologies: pressurized metered-dose inhaler (pMDI), dry power inhaler (DPI), soft-mist inhaler (SMI), nebulizer, and nasal spray Green propellant innovations Experience bringing complex generics to market Expansive capabilities across state-of-the-art facilities […]
Learn MoreOptimizing spray formation of low GWP metered dose inhalers – Unlocking product performance understanding
In response to growing concerns about sustainability, switching to more environmentally friendly pMDI propellants has become a high priority for the pharmaceutical industry. Since inhalation devices contribute 3% of global emissions, this is an important step. In addition to being a part of a greener future, transitioning to a low GWP propellant now positions companies […]
Learn MoreMissed ss at DDL? Unlock pulmonary & nasal insights with our presentations
Kindeva Drug Delivery presented cutting-edge advancements in pulmonary & nasal development and manufacturing at the Drug Delivery to the Lungs (DDL) conference on December 6-8, 2023. Across eight posters and podium presentations, we highlighted strategies for switching to green propellant formulations while preserving product performance, among a variety of other topics. If you missed any […]
Learn MoreThe pMDI dilemma: managing patient needs, human health, and the planet
Learn MoreGreen propellants: The impact of proposed regulations
Learn MoreLet’s transform tomorrow together
Every patient deserves a brighter tomorrow. As your strategic partner, we are dedicated to building your lasting legacy and helping you fast-track healthier tomorrows. You dream it, we deliver it.