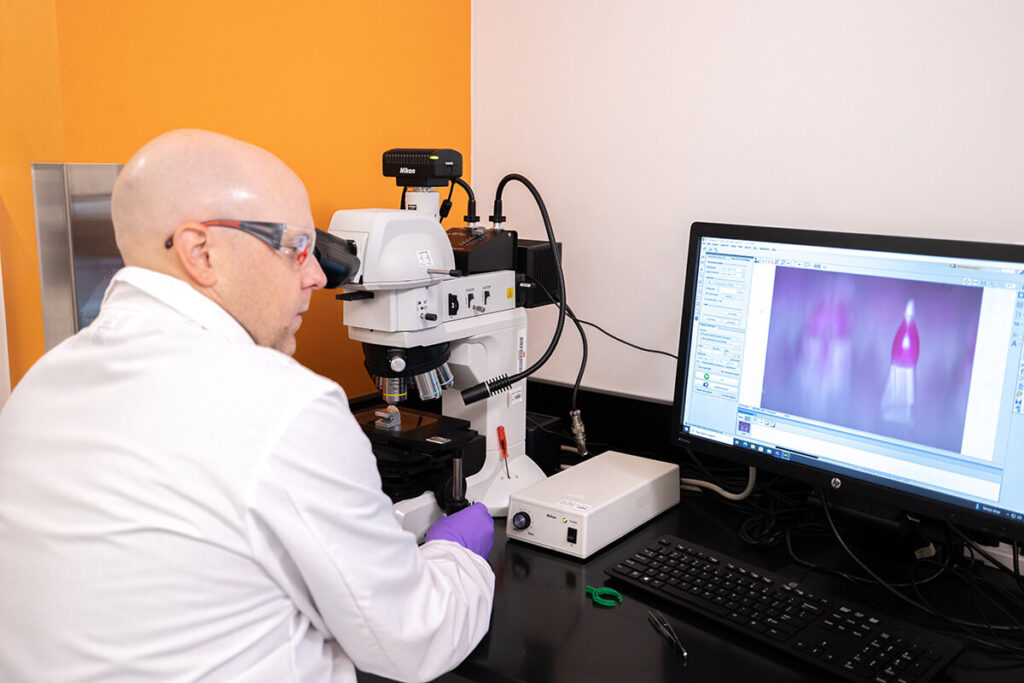
Manufacturing
More TomorrowsTM
Helping you fast-track healthier futures.
As a purpose-fueled drug delivery CDMO, we leverage our specialist injectable, inhalation and dermal expertise to accelerate your product’s journey and make tomorrow’s possibilities a reality.
Innovative CDMO solutions
From development to commercial manufacturing and beyond, amplify your product’s impact with our exceptional-by-design CDMO solutions.

Connect with us
Our Bridgeton facility has over 155,000 sq. ft. of dedicated aseptic operations space, including state-of-the-art laboratories and formulation suites, alongside almost 11,000 sq. ft. of fill suites to ensure the highest levels of quality and patient safety.
We’re hiring
Join us and become part of a CDMO dedicated to advancing our clients’ projects, ambitions and our industry.
Our legacy of innovation
History of Kindeva
Since 1956, we have been developing technologies that meet the demands of today and deliver the possibilities of tomorrow.

2024
Developing low-GWP inhalers
Developing low-GWP inhalers and planning the opening of one of the first commercial-scale green propellant lines for filling inhalers using propellants with up to 99.9% lower GWP than current options.

2012
Invented the CFC-free nasal MDI
Invented the CFC-free nasal MDI, a more environmentally sound device for drug delivery capable of bypassing the blood-brain barrier.

2010
Developed the first commercially available dose counter
Developed the first commercially available dose counter, ensuring patients knew the number of inhaler actuations remaining so they could always be prepared.

2009
Created the TruJect™ platform
Created the TruJect™ platform and introduced the next generation of easy and reliable single-chamber autoinjectors, widely used to deliver a variety of medications.

2005
Solid microneedle array patch development
Solid microneedle array patch development, creating accurate, reliable intradermal delivery with the potential to eliminate cold-chain storage and enhance immunogenicity and efficacy.
2002
Invented the BinaJect® platform
Invented the BinaJect® platform, bringing an industry-leading dual-chamber autoinjector option to market with enhanced stability and bioavailability.

1995
Launched the CFC-free MDI
Launched the CFC-free MDI and set the standard for future care options developed to help safeguard the environment.

1989
Developed the breath-actuated inhaler
Developed the breath-actuated inhaler, a lifesaving option for individuals with hand-breath coordination problems that make traditional inhalers difficult to use.

1970
Launched the drug-in-adhesive patch
Launched the drug-in-adhesive patch, providing a noninvasive method for delivering a drug over an extended timeframe.

1970
Invented the ComboPen® platform
Invented the ComboPen® platform to deliver medical countermeasures, which fueled autoinjector innovations for anaphylaxis.

1959
Created the emergency use autoinjector
Created the emergency use autoinjector, laying the groundwork for the ongoing refinement of self-administered injectables currently used by millions of people worldwide.

1956
Invented the pressurized metered‑dose inhaler (pMDI)
Invented the pressurized metered‑dose inhaler (pMDI) and kick-started the evolution of inhaled therapies by developing an easy-to-use device for patients around the globe.
Let’s transform tomorrow together
Every patient deserves a brighter tomorrow. As your strategic partner, we are dedicated to building your lasting legacy and helping you fast-track healthier tomorrows. You dream it, we deliver it.
Latest at Kindeva
Tomorrow’s in the making at Kindeva. Explore our latest insights and expert resources to advance your drug delivery development and manufacturing.
The next leap in skin-based drug delivery: How dermal delivery platforms are transforming tomorrows for patients
Skin-based drug delivery is gaining attention as patients and healthcare systems look for treatments that support at-home administration and remove the need for needle-based injections. As this shift accelerates, developers are investing in technologies that improve usability without compromising performance. These platforms are opening new possibilities for therapies that rely on reliable, patient-preferred delivery. A […]
Learn MoreWebinar | A Bridge to the Future of Aseptic Manufacturing: A Pharma 4.0 Case Study
Growing demand for high-quality sterile fill finish capacity is putting pressure on development timelines and supply programs across the industry. As expectations for Annex 1 alignment, agility and data-driven control rise, partners need facilities designed to keep pace with evolving requirements. In this on-demand webinar, Chad Hafer, Director of Technical Operations, Aseptic Fill Finish at […]
Learn MoreCareer journeys blog 3: Lauren Harrison
In this story, we meet Lauren Harrison, a formulation scientist who began her path as an apprentice and has spent the last decade building a career rooted in curiosity, hands-on learning and collaboration.
Learn MoreNavigating the transition to next-generation propellants for pMDIs | On-demand webinar
Regulators, health systems and patients expect meaningful progress on sustainability. For pressurized metered dose inhalers (pMDIs), this means planning the transition to low global warming potential (low-GWP) next-generation propellants (NGPs) now, not at the end of the decade. In this webinar with Pharmaceutical Technology, Craig Somerville, Senior Vice President of Kindeva’s pMDI business unit, shares […]
Learn MoreBeyond the contract: Driving transformational partnerships with manufacturing… and more
Achieving scientific breakthroughs is just one hurdle in today’s drug development and manufacturing process aimed at improving patient outcomes. Bringing a new therapy to market requires meticulous execution and clear communication at every step. Unfortunately, even the most groundbreaking scientific advancements can be delayed or even entirely abandoned due to poor performance from partners. This […]
Learn MoreWhy Kindeva: Analytical and regulatory services
Overcome complex analytical and regulatory challenges with a partner dedicated to your product’s success. Our specialist expertise helps you navigate testing, compliance, and quality to advance your project from concept to commercialization. Download our one-pager for a closer look at our integrated approach, including: An overview of our comprehensive, phase-appropriate analytical services. How our regulatory […]
Learn MoreCareer journeys blog 2: Oliver Ingham
In this latest installment, we meet Oliver Ingham, an analytical chemist whose curiosity and problem-solving skills have shaped his journey from academic research to coordinating analytical development services at Kindeva.
Learn MoreCareer journeys blog 1: Holly Dowdle
When Holly Dowdle first joined Kindeva as a student intern, she experienced a workplace that valued both curiosity and collaboration. It was an environment where asking questions was encouraged and development was part of everyday work, not something that happened on the sidelines.
Learn MoreA bridge between design and delivery: Exploring sterile fill finish integration for injectable product success
The global sterile injectable contract manufacturing market is experiencing rapid growth, driven by increasing demand for advanced biologics, glucagon-like peptide-1 (GLP-1) therapies and other injectables. These products pair drugs with delivery devices to support patient-centric care, but their manufacturing presents unique challenges. Programs often require specialized environments, such as cold-chain storage and low-volume fills, and […]
Learn More